Interested in more information? You can watch videos, download guides, connect with WATCHMAN Ambassadors and more.
Find out if you're a candidate for the WATCHMAN Implant as an alternative to blood thinners.
Already took the quiz? Return to your personalized experience.
A safe, minimally-invasive, one-time implant
The WATCHMAN Implant is placed into your heart in a one-time procedure under anesthesia. Patients commonly stay in the hospital for a day or less. It’s a permanent device that doesn’t have to be replaced and can’t be seen or felt outside the body.
To place the WATCHMAN Implant, your doctor makes a small cut in your upper leg and inserts a narrow tube. The WATCHMAN Implant is guided through this tube into the left atrial appendage (LAA) of your heart. After the procedure, your own heart tissue will grow over the device, permanently sealing off the LAA and forming a barrier to blood clots.
 Small cut in upper leg
Small cut in upper leg
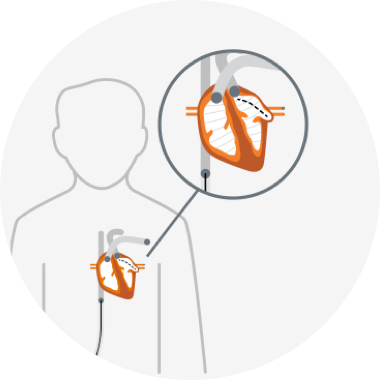 WATCHMAN Implant guided to heart
WATCHMAN Implant guided to heart
 Stay one day or less
Stay one day or less
 Tissue regrows, LAA sealed
Tissue regrows, LAA sealed
Due to the risk of having a medical procedure, patients should not be considered for the WATCHMAN Implant if they are doing well and expect to continue doing well on blood thinners.
Getting the WATCHMAN Implant
Hear what the procedure is like from people who received the WATCHMAN Implant.
"Now I have no restrictions whatsoever. I can do whatever I want to do. I can walk as much as I want to. I can do anything I want to. So that's a really, really great feeling."
One-time procedure.
One-time cost.
Determine your cost and coverage
Compared to the ongoing expenses of blood thinners, the WATCHMAN Implant is a one-time procedure with a one-time cost. This means you can save money over time.
Medicare & Medicare Advantage
The WATCHMAN Implant is covered for eligible Medicare and Medicare Advantage patients who meet the specified national coverage criteria. The estimated out-of-pocket cost for many people covered by Medicare is no more than $2,607.*
Commercial Payers
The WATCHMAN Implant is also broadly covered by commercial insurers. Commercial insurers may choose to follow the Medicare coverage criteria or establish their own coverage criteria. Additionally, commercial insurers may require successfully negotiated prior authorization (PA) of the procedure between the health care provider and the plan before it takes place.
Keep in mind that:
You may need prior authorization or pre-certification from your plan provider.
Your doctor's office will likely work with you to complete this process. If your coverage is denied, you can work with your doctor on an appeal.
If you have questions or concerns about your coverage, please reach out directly to your insurance carrier. They should be able to help answer any questions about your plan-specific coverage.
Already took the quiz? Return to your personalized experience.
After the procedure
Following the procedure, your doctor will prescribe a medication regimen that is right for you. Typically, this will be one of two options:
Option A
You may be prescribed a short-term blood thinner (anticoagulant), typically for 45 days. Then your doctor may stop blood thinners and put you on an anti-platelet and aspirin until 6 months post-procedure. After that, you’ll continue to take aspirin on an ongoing basis.

Option B
You may be prescribed an anti-platelet medicine called clopidogrel (also known as Plavix®) and aspirin for 6 months. At your 6-month appointment, your doctor may stop the antiplatelet medicine and you’ll continue to take aspirin on an ongoing basis.

These medications are taken until your left atrial appendage is completely closed off. Whichever option is chosen, it’s important that you discuss your medications with your doctor and do not change or modify your medications or dosages unless prescribed by your doctor.
Support for your next steps

Speak to an Education Specialist
WATCHMAN Education Specialists are trained professionals with healthcare experience. They're here to help answer your questions.
Monday to Friday, 8 a.m. to 5 p.m. CT
Next steps
*A typical Medicare patient in 2024 is estimated to pay no more than $2,607. Cost includes: Pre-screen TEE, implant procedure, professional physician fees, and post-implant OAC therapy and TEE.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.
Content on this web page is for Informational Purposes only and does not constitute medical advice and should not be used for medical diagnoses. Boston Scientific strongly recommends that you consult with your physician on all matters pertaining to your health or to address any clinical/medical questions.
Important Safety Information
The WATCHMAN FLX and WATCHMAN FLX Pro Devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include, but are not limited to, accidental puncture of the heart causing fluid to collect around the heart possibly leading towards the need for an additional procedure, allergic reaction, anesthesia risks, altered mental status or confusion after procedure, arrhythmias (irregular heartbeats), bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, chest pain/discomfort, congestive heart failure, renal failure, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, bruising at the catheter insertion site, groin pain, anemia (reduced red blood cells requiring transfusion), hypotension, infection/pneumonia (example: in or around your heart or lungs), misplacement of the device, improper seal of the appendage or movement of device from appendage wall, clot formation on the device, blood clot or air bubbles in the lungs or other organs, stroke, transient ischemic attack (temporary stroke-like symptoms), cranial bleed (bleeding in or around your brain), thrombosis (blockage of a blood vessel or vein by a clot) and in rare cases death can occur.
Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the device. SH-2109508-AA



