Shaping the future of LAAC therapy
Built on the proven performance of the world’s most studied LAAC device, the WATCHMAN FLX™ Left Atrial Appendage Closure Device1, the WATCHMAN FLX Pro Device features HEMOCOAT™ Technology to help promote faster, more complete endothelialization.2
Transforming AFib care for more patients
Breakthrough studies like the OPTION Clinical Trial and the SIMPLAAFY trial—inspired by early HEMOCOAT technology—are shaping a new era in LAAC and atrial fibrillation (AFib) care. These studies drive transformative outcomes, explore innovative post-implant drug regimens, and improve patient outcomes.
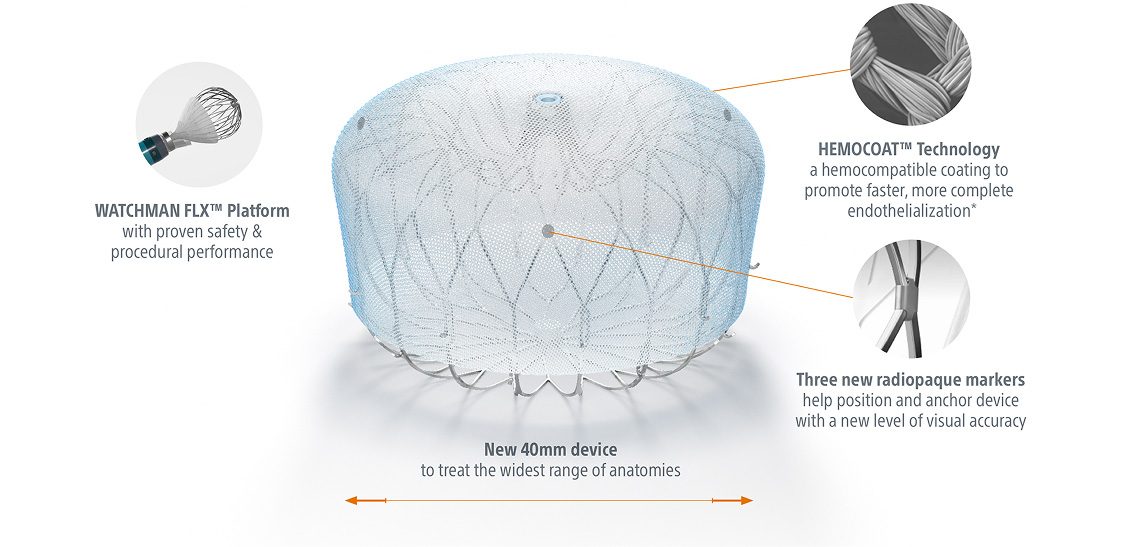
WATCHMAN FLX Pro LAAC Device
The WATCHMAN FLX Pro Device is designed with three first-ever features: new HEMOCOAT Technology designed to improve the healing process, radiopaque markers for precise device placement, and a new 40mm size for larger appendages.
*Enhanced healing demonstrated in pre-clinical model with no anticoagulation
Proven in performance, optimized for healing.
The WATCHMAN FLX Pro builds on the most studied and implanted LAAC device in the world by taking the next step forward in features and benefits.
Coated for controlled healing
HEMOCOAT Technology, a durable, thromboresistant coating, is designed to reduce inflammation for faster, more complete endothelialization.2
Established
PVDF-HFP has a long history of safe use on permanently implanted, blood-contacting medical devices.3
Stable
A durable, thin coating encapsulates the device’s healing surface, maintaining pore size and the mechanical performance of the WATCHMAN FLX platform (<1µm).4
Proven
Demonstrates impressive performance in a challenging preclinical model.4
How HEMOCOAT technology works to heal
Testing in pre-clinical model has demonstrated faster, more controlled healing.2
Enhanced visibility for greater precision
Three new radiopaque markers help improve visual accuracy, for precise device positioning and anchoring.
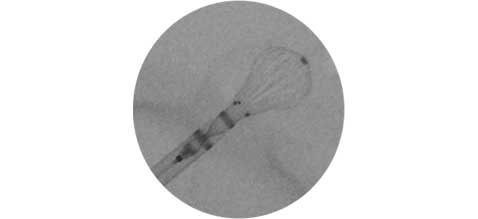
Enhance deployment precision
57% increased visibility for more accurate device positioning5
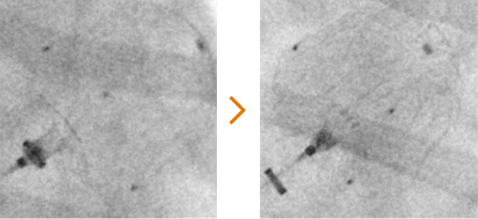
Ensure device stability
Improved assessment of device anchoring when performing tug test

Enable confident release
Improved visualization of device orientation and alignment
Close with confidence
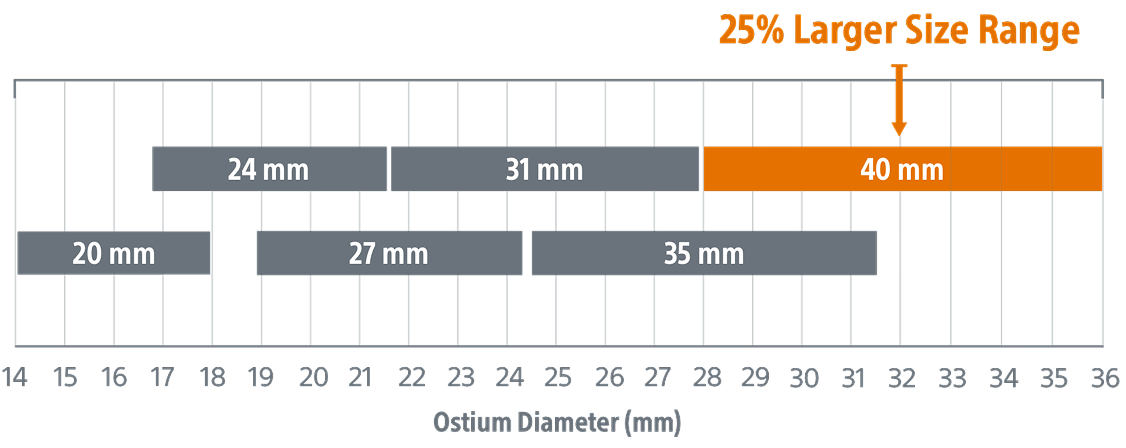
A new 40mm size to help treat larger appendages

Expanded size, same trusted performance
- Same performance
- Same compression range (10‑30%)
- Same depth requirement

*Devices not shown to scale
Increased flexibility for post-implant drug regimens
As of July 2025, the WATCHMAN FLX Pro IFU now incorporates key clinical findings from the OPTION trial and SURPASS registry. These FDA-approved updates ensure that the latest data is reflected in treatment guidelines, expanding clinical flexibility and post-implant medication options for your WATCHMAN patients.
Key takeaways from the label updates include:
An updated indication for NVAF patients, post-catheter ablation, that
- removes the need for suitable rationale to seek non-pharmacologic alternatives to oral anticoagulants,* and
includes a specific 3-month post-implant drug regimen aligned to OPTION protocol.
* While the WATCHMAN FLX Pro device label has an updated indication, the National Coverage Determination has not been updated. The patient must be deemed unable to take long-term oral anticoagulation and meet all criteria in NCD 20.34 to be eligible for coverage.
- A new 45-day NOAC alone (followed by DAPT) drug regimen option for standalone LAAC patients.
Here’s a quick view of the expanded regimen:
Post-implant drug regimen options
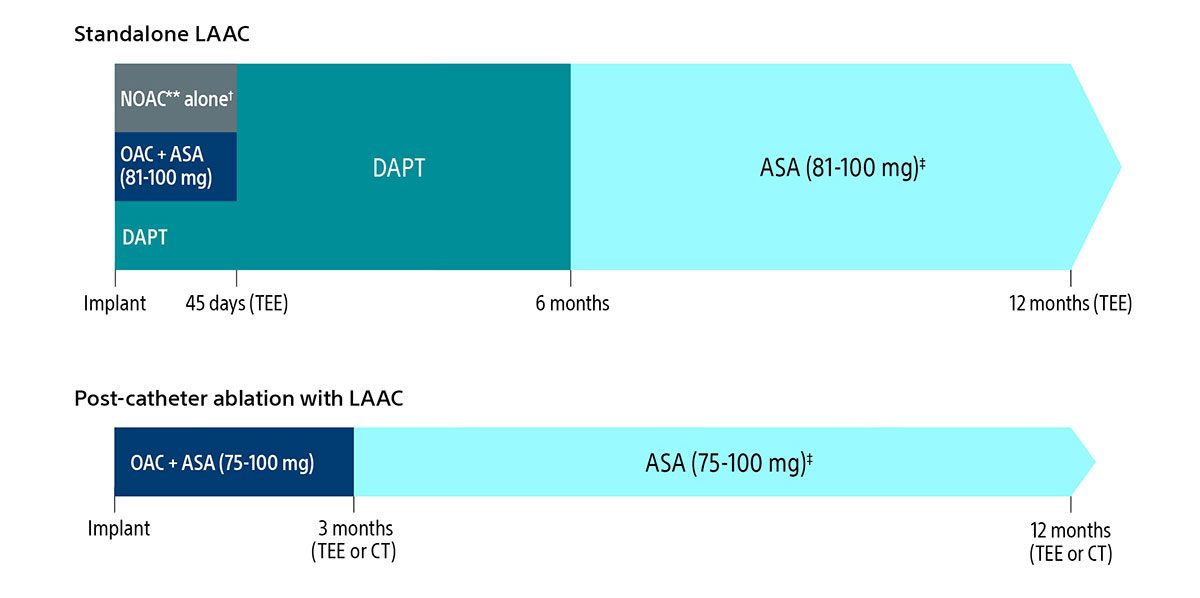
** Excludes Warfarin.
† Pre-procedure ASA is per physician discretion if the physician intends to prescribe NOAC alone for the patient post-procedure.
‡ Continued indefinitely.
As always, you should exercise clinical judgment based on individual patient characteristics in determining the most appropriate use of anti-thrombotic drugs for the post-implant medication regimen.
The WATCHMAN FLX Pro Device is the only LAAC device that does not require an overnight stay after the procedure. Clinical outcomes support its safety and efficacy in preventing thrombosis and reducing stroke risk.
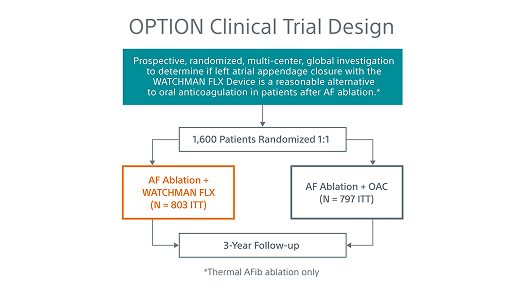
Learn about the OPTION randomized clinical trial
References
1. Kar, S., et al, Circulation, 2021
2. Saliba et al. JACC: Clinical Electrophysiology, May 2023. Bench testing or pre-clinical study results may not necessarily be indicative of clinical performance. N=12 in a pre-clinical canine study.
3. Wagner et al., Biomaterials Science: An Introduction to Materials in Medicine, 4th Edition, 2020.
4. Boston Scientific Data on file.
5. Bench study performed under CT by Boston Scientific. Data on file.
