Consistent with the overall primary endpoint outcomes, the WATCHMAN FLX device demonstrated equal thromboembolic protection to OAC (95% DOACs) at 36 months, while significantly reducing bleeding risks irrespective of concomitant or sequential LAAC following an ablation, providing an option for patients seeking an alternative to long-term anticoagulation regardless of the timing of LAAC relative to the ablation.
Positive Primary Endpoints Reaffirmed
Listen to Dr. Walid Saliba (Cleveland Clinic Principal Investigator) discuss the concomitant and sequential cohort subanalysis.
Study design and patient disposition
The objective of the OPTION trial was to determine if LAAC with WATCHMAN FLX is a reasonable alternative to OAC following AF ablation. Consistent with this objective, this subanalysis compared outcomes among the concomitant and sequential cohorts of the WATCHMAN FLX arm to those randomized to oral anticoagulation following AF ablation.

Primary Efficacy Endpoint
Stroke, all-cause mortality and systemic embolism
Primary Safety Endpoint
Non-procedural bleeding (ISTH major bleeding and clinically relevant non-major bleeding)
- Concomitant: AF ablation ± WATCHMAN FLX implantation within 10 days of randomization
- Sequential: AF ablation 90 to 180 days prior to randomization
- The planned ablation timing, concomitant or sequential, was not randomized but must have been specified by the investigator prior to randomizing the subject
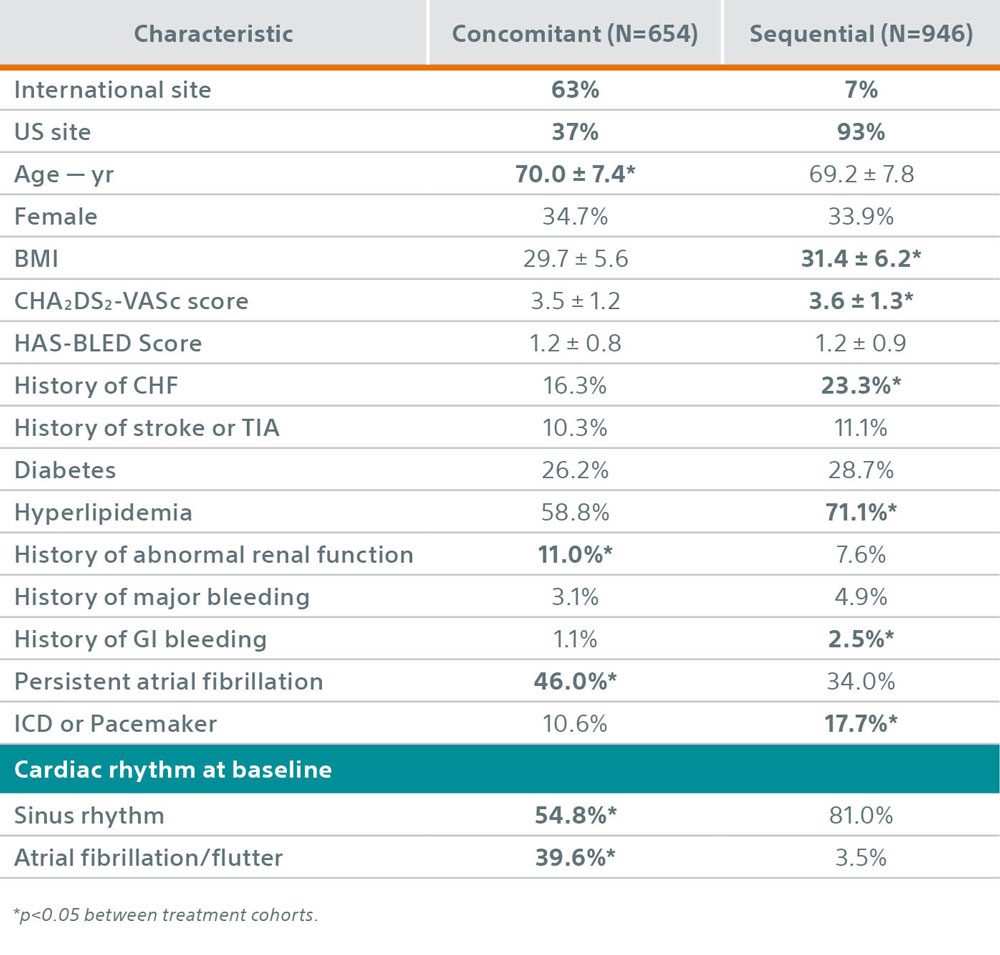
Key Baseline Characteristics – Concomitant & Sequential Subgroups
Irrespective of treatment (WATCHMAN FLX vs. OAC) arm
- The planned ablation timing, concomitant or sequential, was not randomized but must have been specified by the investigator prior to randomizing the subject.
- Patient characteristics between the concomitant and sequential cohorts were unbalanced, suggesting the subgroups represent disparate patient populations reflected by potential selection bias driven by baseline characteristics that may influence perceived suitability for either a concomitant or sequential procedure.
Concomitant Cohort Outcomes
Concomitant Cohort Primary Endpoints
Consistent with the overall primary outcomes, concomitant LAAC with WATCHMAN FLX following AF ablation showed similar efficacy to OAC, while demonstrating a statistically significant 44% risk reduction in bleeding events at 36 months.


Concomitant Cohort 3-Year Clinical Outcomes
Concomitant LAAC with WATCHMAN FLX demonstrated similar long-term stroke protection to anticoagulation, including a 1.6% ischemic stroke rate at 3 years.

Concomitant Cohort CEC-Adjudicated Procedural Events
Sensitivity analyses were performed in the on-treatment population where patients in each treatment group were tested according to LAAC or OAC treatment received and if they were compliant to protocol medication at ≥80% until a primary or secondary event or end of study.
Ablation + WATCHMAN FLX
Procedural Event Rate
(within 7 days)
Ablation [Only] + OAC
Procedural Event Rate
(within 7 days)
Concomitant LAAC with WATCHMAN FLX at the time of ablation resulted in similar procedure related events (2.1%) compared to the ablation-only control arm (2.7%), therefore resulting in no increase in procedural risk with the addition of the WATCHMAN FLX procedure to ablation.
Sequential Cohort Outcomes
Sequential Cohort Primary Endpoints
Consistent with the overall primary outcomes, sequential LAAC with WATCHMAN FLX following AF ablation showed similar efficacy to OAC, while demonstrating a statistically significant 62% risk reduction in bleeding events at 36 months.


Sequential Cohort 3-Year Clinical Outcomes
Sequential LAAC with WATCHMAN FLX demonstrated similar long-term stroke protection to anticoagulation, including a 0.9% ischemic stroke rate at 3 years.

WATCHMAN FLX is an FDA approved device being studied for an expanded indication as a first line therapy vs NOAC for NVAF patients. The use of WATCHMAN or WATCHMAN FLX as a first-line therapy for stroke risk reduction in NVAF patients is considered investigational.
Caution: Investigational Device. Limited by US law to investigational use only. Not available for sale.
