The primary endpoints showed WATCHMAN FLX™ was equally effective to anticoagulation, with a superior safety profile, which allowed patients to eliminate continuous medication use and significantly reduce bleeding risk while maintaining stroke protection.1
Positive Trial Outcomes!
OPTION Clinical Trial discussion with Dr. Balderrama
Listen as cardiologist Dr. Tadeo Diaz Balderrama discusses the positive results from the OPTION Clinical Trial and how the outcomes may impact referring practices.
OPTION Clinical Trial Design

Primary Efficacy Endpoint
Stroke, all-cause mortality and systemic embolism
(NON-INFERIORITY)
Primary Safety Endpoint
Non-procedural bleeding (ISTH major bleeding and clinically relevant non-major bleeding)
(SUPERIORITY)
Secondary Safety Endpoint
ISTH major bleeding (including procedural bleeding)
(NON-INFERIORITY)
Key Inclusion Criteria:
- The subject has a calculated CHA2DS2-VASc score of 2 or greater for males or 3 or greater for females.
- Underwent a prior catheter ablation procedure for non-valvular AF between 90 and 180 days prior to randomization (sequential) or is planning to have clinically indicated catheter ablation within 10 days of randomization (concomitant).
Key Baseline and Procedural Characteristics (ITT)
Patient characteristics were well balanced between groups, while LAAC success was achieved in 98.8% of patients in the WATCHMAN FLX arm.

LAAC success** reaffirms the industry-leading procedural success of the WATCHMAN FLX procedure observed in the PINNACLE FLX pivotal trial.1
OPTION Primary Endpoint Outcomes
Primary Efficacy Endpoint
The trial met the primary efficacy endpoint of all-cause mortality, stroke or systemic embolism at 36 months, with the WATCHMAN FLX device demonstrating statistical non-inferiority to OAC (5.3% vs. 5.8%; P<0.0001).

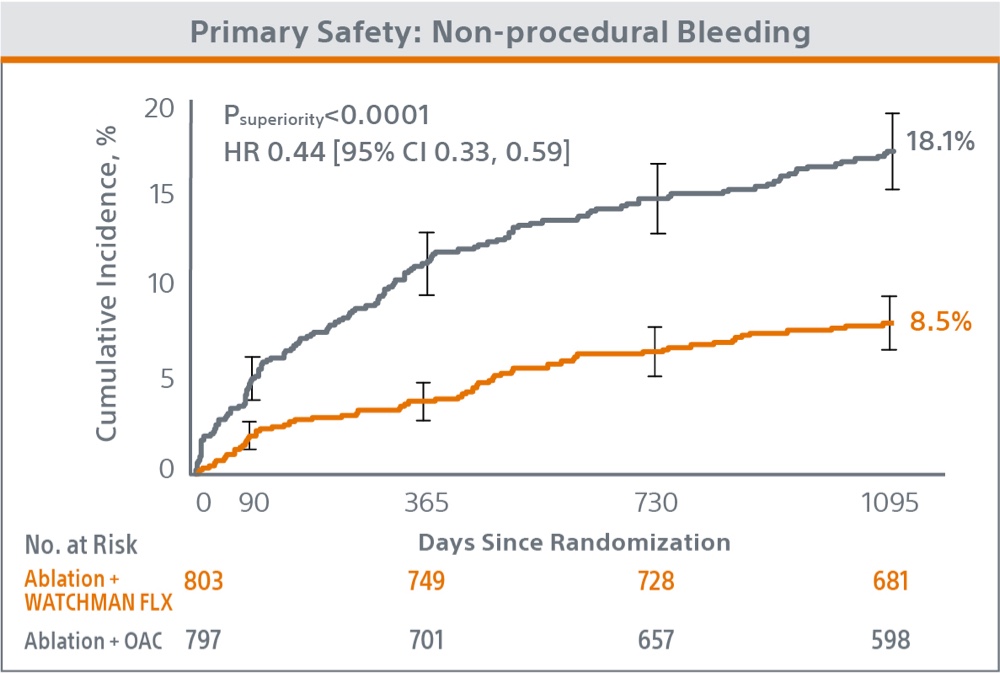
Primary Safety Endpoint
The trial met the primary safety endpoint of non-procedural major bleeding or clinically relevant non-major bleeding at 36 months, with the WATCHMAN FLX device demonstrating statistical superiority to OAC (8.5% vs. 18.1%; P<0.0001).
Primary Efficacy Endpoint Components (ITT)
WATCHMAN FLX demonstrated similar long-term efficacy outcomes compared to anticoagulation, including a 1.6% all-stroke and 1.2% ischemic stroke rate.
3-Year Primary Efficacy Endpoint Outcomes

Primary Safety Endpoint (Including Procedural Bleeding)
Even with the inclusion of procedural bleeding, WATCHMAN FLX demonstrated a 50% relative reduction in ISTH major and clinically relevant non-major bleeding at 36 months (9.5% vs. 18.1%; P<0.0001).

The 50% relative reduction (HR 0.50 [95% CI 0.38, 0.66]) in ISTH bleeding (including procedural bleeding) at 36 months further reaffirms superiority of WATCHMAN FLX for the primary safety endpoint.
Primary and Secondary Endpoint On-Treatment Sensitivity Analysis
Sensitivity analyses were performed in the on-treatment population where patients in each treatment group were tested according to LAAC or OAC treatment received and if they were compliant to protocol medication at ≥80% until a primary or secondary event or end of study.

Explore the proven safety and efficacy outcomes
1. Wazni O, et al. Randomized Comparison of Left Atrial Appendage Closure with Oral Anticoagulation After Catheter Ablation for Atrial Fibrillation. Late Breaking Clinical Trial, American Heart Association 2024.
WATCHMAN FLX is an FDA approved device being studied for an expanded indication as a first line therapy vs NOAC for NVAF patients. The use of WATCHMAN or WATCHMAN FLX as a first-line therapy for stroke risk reduction in NVAF patients is considered investigational.
Caution: Investigational Device. Limited by US law to investigational use only. Not available for sale.
